Fahn Tolosa Marin Tremor Rating Scale Pdf Page
Fahn, Tolosa, Marin Tremor Rating Read more about tremor, unable, mildly, moderately, severely and markedly.

Adobe Flash Player is required to view this feature. If you are using an operating system that does not support Flash, we are working to bring you alternative formats.
Original Article A Randomized Trial of Focused Ultrasound Thalamotomy for Essential Tremor W. Jeffrey Elias, M.D., Nir Lipsman, M.D., Ph.D., William G. Ondo, M.D., Pejman Ghanouni, M.D., Ph.D., Young G. Kim, M.D., Ph.D., Wonhee Lee, M.D., Ph.D., Michael Schwartz, M.D., Kullervo Hynynen, Ph.D., Andres M.
Lozano, M.D., Binit B. Shah, M.D., Diane Huss, D.P.T., N.C.S., Robert F. Dallapiazza, M.D., Ph.D., Ryder Gwinn, M.D., Jennifer Witt, M.D., Susie Ro, M.D., Howard M. Eisenberg, M.D., Ph.D., Paul S. Fishman, M.D., Ph.D., Dheeraj Gandhi, M.D., M.B., B.S., Casey H. Halpern, M.D., Rosalind Chuang, M.D., Kim Butts Pauly, Ph.D., Travis S. Tierney, M.D., Ph.D., Michael T.
Hayes, M.D., G. Rees Cosgrove, M.D., Toshio Yamaguchi, M.D., Ph.D., Keiichi Abe, M.D., Takaomi Taira, M.D., Ph.D., and Jin W.
Chang, M.D., Ph.D. N Engl J Med 2016; 375:730-739 DOI: 10.1056/NEJMoa1600159. Methods We enrolled patients with moderate-to-severe essential tremor that had not responded to at least two trials of medical therapy and randomly assigned them in a 3:1 ratio to undergo unilateral focused ultrasound thalamotomy or a sham procedure. The Clinical Rating Scale for Tremor and the Quality of Life in Essential Tremor Questionnaire were administered at baseline and at 1, 3, 6, and 12 months.
Tremor assessments were videotaped and rated by an independent group of neurologists who were unaware of the treatment assignments. The primary outcome was the between-group difference in the change from baseline to 3 months in hand tremor, rated on a 32-point scale (with higher scores indicating more severe tremor). After 3 months, patients in the sham-procedure group could cross over to active treatment (the open-label extension cohort). Results Seventy-six patients were included in the analysis. Hand-tremor scores improved more after focused ultrasound thalamotomy (from 18.1 points at baseline to 9.6 at 3 months) than after the sham procedure (from 16.0 to 15.8 points); the between-group difference in the mean change was 8.3 points (95% confidence interval [CI], 5.9 to 10.7; P. Figure 1 Tremor Scores.
Panel A shows tremor scores at baseline and throughout the 12-month study period. The change from baseline to 3 months in the tremor score for the hand contralateral to the thalamotomy, the primary outcome measure, was derived from eight items on the Clinical Rating Scale for Tremor (CRST; scores range from 0 to 32, with higher scores indicating more severe tremor). At 3 months, the mean score was reduced by 47% in the group assigned to unilateral focused ultrasound (FUS) thalamotomy, as compared with a reduction of 0.1% in the group assigned to the sham procedure (P. Figure 2 Functional Activities of Daily Living and Quality of Life. Panel A shows total disability scores, which were significantly improved at 3 months (P. Essential tremor, the most common movement disorder, is characterized by a distinctive postural and intention tremor typically affecting the hands more than the legs, trunk, head, or voice. Essential tremor does not shorten life expectancy, but it can affect quality of life, functional activities, mood, and socialization.
Class I evidence exists for propranolol and primidone as first-line medications that reduce tremor by approximately 60% in 50% of patients. If resistance to medications develops or side effects are unacceptable, neurosurgical intervention is considered, primarily targeting the nucleus ventralis intermedius of the thalamus, a component of tremor circuitry that connects the cerebellum with cortical motor pathways. Two surgical therapies, radiofrequency thalamotomy and deep-brain stimulation, effectively suppress tremor, but relatively few patients choose surgery because of perceived invasiveness from the burr holes and intracerebral electrodes.
The use of ultrasound energy for the creation of discrete intracranial lesions (hereafter referred to as lesioning) has been of interest since the middle of the 20th century. Initial procedures required craniotomy to establish an acoustic window for the treatment of movement disorders and psychiatric conditions. The subsequent introduction of phased-array transducers eliminated the need for a craniotomy, and high-resolution imaging allows real-time, image-guided lesioning (see Fig. S1 in the, available with the full text of this article at NEJM.org). Prospective pilot trials of focused ultrasound thalamotomy with magnetic resonance imaging (MRI) guidance in patients with essential tremor have shown reductions in hand tremor, improvements in quality of life, and minimal procedural morbidity. Here we describe the results of a prospective, sham-controlled trial of MRI-guided focused ultrasound thalamotomy for the treatment of medication-refractory essential tremor. Trial Design and Oversight In this double-blind trial conducted at eight international centers, we randomly assigned patients in a 3:1 ratio to undergo focused ultrasound thalamotomy or a sham procedure in which no acoustic energy was delivered.
The primary study end point was the change in tremor from baseline to 3 months, analyzed on the basis of videotaped assessments. After 3 months, patients in the sham-procedure group could cross over to active treatment (Fig. Representatives of the manufacturer of the focused ultrasound system used in the study (InSightec) provided study oversight and technical support and obtained national regulatory permissions. Free Download Start Orb Studio.
Independent institutional approval of the study was obtained by the research team at each participating center, and all patients gave written informed consent. Clinical oversight of the trial was provided by the principal investigator and an independent data and safety monitoring board. The authors vouch for the veracity and completeness of the data and data analyses. The first author wrote the first draft of the manuscript, and all authors made the decision to submit the manuscript for publication. The study was conducted with fidelity to the study, which is available at NEJM.org. Patients Patients with essential tremor, diagnosed by a neurologist specializing in movement disorders, were enrolled on the basis of eligibility criteria that have been described previously.
Briefly, patients were eligible if they had a postural or intention tremor of the hand that was moderate to severe (defined by a score of ≥2 on the Clinical Rating Scale for Tremor [CRST; scores range from 0 to 4 per component assessed and higher scores indicate more severe tremor]) and disabling (defined by a score of ≥2 on any of the eight items in the disability subsection of the CRST [scores range from 0 to 4 per item, and higher scores indicate greater disability]). Additional eligibility criteria were tremor that was refractory to at least two trials of medical therapy, including at least one first-line agent (propranolol or primidone).
For patients receiving concurrent medical therapy, medication doses had to be stable for 30 days before randomization. Patients were excluded if they had a neurodegenerative condition, unstable cardiac disease, coagulopathy, risk factors for deep-vein thrombosis, severe depression (defined by a score ≥20 on Patient Health Questionnaire 9 [scores range from 0 to 27, with higher scores indicating more severe depression]), or cognitive impairment (defined by a score of. Focused Ultrasound Thalamotomy The details of focused ultrasound thalamotomy have been described previously. Briefly, patients were placed in a stereotactic head frame that was coupled to an MRI-compatible ultrasound transducer. After stereotactic targeting with the use of MRI, acoustic energy was sequentially titrated to temperatures sufficient for tissue ablation (approximately 55 to 60°C).
Each brief sonication was monitored with magnetic resonance thermometry, and the patient was clinically assessed for tremor reduction and adverse effects (for details, see the description in the ). For patients randomly assigned to undergo a sham procedure, an identical procedure was performed with a randomized number of sonications for which the acoustic power was disengaged so that no acoustic energy was delivered to the brain.
Only the treatment team was aware of the group assignments; patients and assessors were unaware of the assignments. Outcome Assessments Tremor assessments, based on the CRST, were performed at each site by a movement-disorder specialist, and functional status was determined on the basis of the rating for the disability subsection (Part C) of the CRST, as well as the disease-specific, self-reported Quality of Life in Essential Tremor Questionnaire (QUEST).
Tremor evaluations were videotaped for primary analysis by an independent core group of neurologists (Tremor Research Group) at baseline and at 1, 3, 6, and 12 months after treatment. The primary efficacy outcome measure was defined as the change from baseline to 3 months in the tremor score for the hand in the thalamotomy group as compared with the sham-procedure group. The tremor score (on a scale ranging from 0 to 32, with higher scores indicating more severe tremor) was derived from the CRST, Part A (three items: resting, postural, and action or intention components of hand tremor), and the CRST, Part B (five tasks involving handwriting, drawing, and pouring), in the hand contralateral to the thalamotomy. The three prespecified secondary efficacy measures were functional limitations in daily activities, measured according to eight items in the disability subsection of the CRST (maximum overall score, 32; higher scores indicate greater disability); quality of life, assessed with the QUEST at 3 months; and the durability of the reduction in hand tremor at 12 months. We also performed a post hoc analysis of total tremor scores (maximum overall score for the most severe tremor, 152 points without supine assessments). Safety was assessed throughout the study on the basis of reported adverse events.
MRI was performed immediately after the study procedure and at 12 months. Blinding The study participants and the neurologist at each site were unaware of the treatment assignments throughout the first 3 months, and the primary assessors of the videotaped tremor evaluations were not involved in the study treatments and were unaware of the treatment assignments and the side that was treated (left vs.
Since the patients’ heads were not covered, the assessors could see whether the videotapes showed preoperative or postoperative tremor evaluations; however, they could not determine whether the videotapes were taken 1, 3, 6, or 12 months after treatment. Statistical Analysis We calculated the sample size from pilot-study observations, accounting for a potential dropout rate of 20%. The null hypothesis was that thalamotomy would be either the same as or inferior to the sham procedure with respect to the percentage improvement in the primary end point. The alternative hypothesis was that thalamotomy would be superior to the sham procedure. Given a sample of at least 60 patients, the study had almost 100% power to show the primary efficacy of thalamotomy, assuming, on the basis of historical results, average improvements of 78% and 4% in the thalamotomy and sham-procedure groups, respectively (standard deviation, 25%). Power calculations were performed with the use of an independent-groups t-test, with a randomization ratio of 3:1 for assignment to thalamotomy versus the sham procedure. The probability of detecting an adverse event rate of 1% was 0.45, and the probability of a 5% rate was 0.95.
The statistical analysis was planned and conducted with the assistance of the biostatistics team at TechnoSTAT. The statistical analysis plan (see the study ) was approved by the Food and Drug Administration (FDA). We used a hierarchical testing design to control for multiple comparisons across the one primary and three prespecified secondary end points. The primary efficacy analysis was confirmed at an alpha level of 0.05, and then each of the three secondary efficacy end points was tested at an alpha level of 0.05. No confirmatory statements were made about other end points. Thus, type 1 error was controlled across all end points tested in this study.
A sensitivity analysis with multiple imputation was planned, but in the primary analysis, only two patients had missing data. Since worst-case and best-case scenarios yielded such similar results, additional imputation was not carried out. A second sensitivity analysis, performed because five patients were found to meet exclusion criteria after randomization, confirmed that their exclusion had no effect on the results of the primary outcome analysis (see the ). The data reported here were locked on September 17, 2015, and the report was finalized on October 14, 2015. Study Participants The 76 patients had a mean (±SD) age of 71.0±8.3 years (range, 47 to 89) and a mean disease duration of 16.8±12.3 years. Most of the patients were men (68%), right-handed (83%), and white (75%), and most had a family history of tremor (72%).
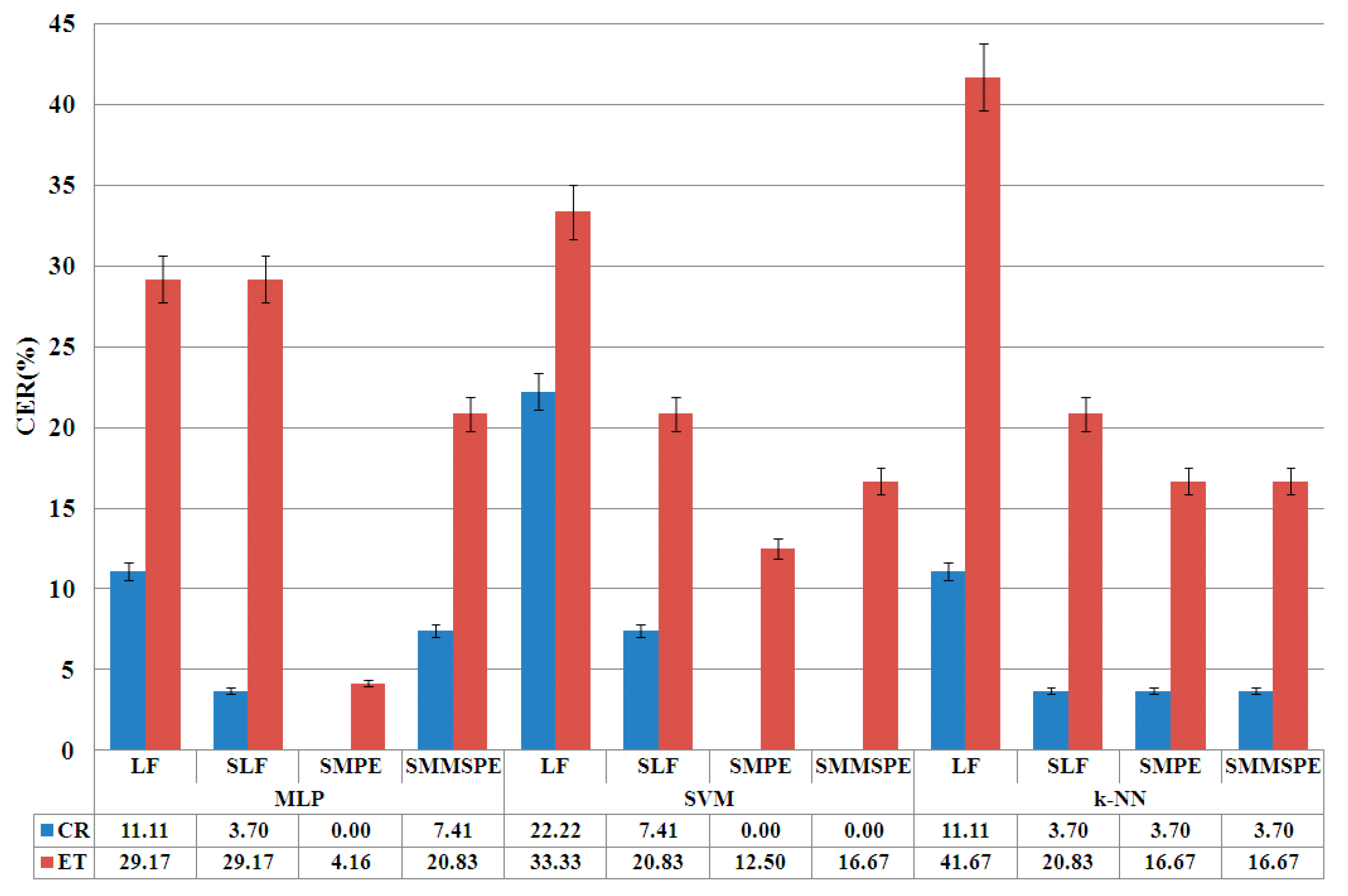
The mean total CRST score for tremor severity was 49.5 (highest possible score, 152). Baseline tremor and demographic characteristics did not differ significantly between the randomized groups ( Table 1 Baseline Demographic and Clinical Characteristics of the Study Participants. Tremor The mean score for hand tremor (highest possible score, 32) improved by 47% at 3 months (from 18.1±4.8 to 9.6±5.1) in the thalamotomy group and by 0.1% in the sham-procedure group (from 16.0±4. Download Civil Engineering Textbooks. 4 to 15.8±4.9).
The between-group difference in the mean change at 3 months, the primary efficacy end point, was 8.3 points (95% confidence interval [CI], 5.9 to 10.7; P. Functional Improvement and Quality of Life Focused ultrasound thalamotomy significantly improved the total disability score from Part C of the CRST at 3 months, as compared with the sham procedure (a 62% reduction in the score from baseline to 3 months [from 16.5±4.6 to 6.2±5.6] vs. A 3% reduction [from 16.0±4.3 to 15.6±4.6], P.
Adverse Events Adverse events associated with focused ultrasound thalamotomy included gait disturbance in 36% of patients and paresthesias or numbness in 38%; these adverse events persisted at 12 months in 9% and 14% of patients, respectively ( Table 2 Adverse Events. The sensory side effects of numbness or paresthesia involved the face (in 8 patients), hand (in 6), or both (in 6), presumably from involvement of the adjacent ventral posterolateral (sensory) nucleus.
One patient had dense and permanent hypesthesia of the dominant thumb and index finger, categorized as a serious adverse event. Gait disturbances also occurred, with ataxia noted on postoperative neurologic examination (in 11 patients [20%]) and at 12 months (in 2 patients [4%]). Subjective unsteadiness was reported by 9 patients (16%), which persisted at 12 months in 3 patients (5%).
Weakness contralateral to the thalamotomy, probably resulting from internal-capsule involvement, occurred in 2 patients, persisting for 6 months in 1 and 12 months in the other. Intraprocedural sensations resolved within seconds after the delivery of acoustic energy ( ). A similar profile of side effects was observed in the unblinded cohort of patients undergoing focused ultrasound thalamotomy (Table S5 in the ). One patient had a transient ischemic attack 6 weeks after undergoing thalamotomy (Table S6 in the ). Sonications For the 56 patients undergoing focused ultrasound thalamotomy, a mean of 18.5±5.2 sonications were administered. The highest-energy sonication for tissue ablation delivered a mean acoustic energy of 14,497.0±6695.7 J (range, 3500 to 34,860), which resulted in a mean peak voxel temperature of 55.6±2.3°C (range, 50.0 to 60.7).
The sham-procedure cohort received an average of 15.3±2.3 sonications, with no energy or heating delivered. In 39 active treatments, intraoperative clinical or imaging feedback led to a mean adjustment in the stereotactic target location by 1.6±1.1 mm (range, 1.1 to 5.5). In 5 patients, the full therapeutic temperature could not be achieved, despite their receiving similar doses of acoustic energy. Survey of Patients and Assessors about Randomized Assignments Special procedures were implemented to ensure blinding of the treatment assignments. Even so, 95% of patients who underwent active treatment and 80% of those who underwent the sham procedure correctly guessed their assignment immediately after the procedure. At the end of the 3-month blinded phase, the correct guesses were 86% and 95%, respectively, with patients accrediting their opinion to the clinical effect of the treatment or lack thereof.
Assessors who reviewed the videotaped examinations at 3 months correctly identified the treatment assignment for 70% of the patients in the active-treatment group and 75% of those in the sham-procedure group, most likely on the basis of the presence or absence of a clinical effect. Discussion In this randomized, controlled trial involving 76 patients with medication-refractory essential tremor, transcranial focused ultrasound thalamotomy significantly reduced hand tremor at 3 months, and the effect persisted during the 12-month study period. This unilateral procedure reduced disability and improved quality of life as measured by a patient questionnaire that is specific for essential tremor. The trial was controlled with a sham procedure, and the results show that tremor reduction was related to treatment, not a placebo effect.
In addition, the Tremor Research Group, a group of experts who were not involved in the treatments, was recruited to objectively evaluate the clinical outcomes from videotape analysis. There was high accountability in this trial, with 97% of patients completing study visits throughout the 3-month primary assessment period, and 91% of the thalamotomy group assessed through 12 months (Fig. Even though the procedure is transcranial and involves no incision or craniotomy, it does create a thalamic lesion, which can result in permanent neurologic deficits. There were 74 neurologic adverse events reported in the 56 patients who underwent active treatment.
The most common side effect was an alteration in sensation, which was reported by 38% of the patients and persisted at 12 months in 14%. Gait disturbance occurred in 36% of patients and persisted at 12 months in 9%.
The incidence of cerebellar deficits such as dysmetria, ataxia, and subjective unsteadiness of gait approached 5% each at 12 months. There were no infectious or hemorrhagic events, but contralateral weakness occurred twice. Qualitatively, the intensity of side effects seemed to peak at approximately 1 week, corresponding to the maximal size of the lesion with perilesional edema. Although randomized, controlled studies of medical therapies have shown tremor reductions in roughly 50% of study participants, these studies were performed at the early stages of the disease. The current trial shows that focused ultrasound thalamotomy can further control tremor when it has become advanced and resistant to medication.
Deep-brain stimulation is currently the surgical standard for medication-refractory essential tremor. Since FDA approval of the procedure in 1997, numerous studies have confirmed that it is highly effective for tremor suppression, but guidelines have classified the findings as level C evidence in the absence of placebo-controlled trials. Deep-brain stimulation has been safely administered for bilateral and axial symptoms. The procedure requires surgical placement of a neurostimulator that can be reversed and adjusted to minimize side effects.
Focused ultrasound thalamotomy is also an invasive intervention, which can result in permanent side effects as a consequence of tissue ablation. A control group of patients undergoing deep-brain stimulation was not included in this trial; the two technologies were not compared. Stereotactic radiofrequency thalamotomy for tremor has been available since the 1950s, with numerous retrospective studies documenting efficacy similar to that of thalamic stimulation. Recently, a prospective, uncontrolled trial of stereotactic radiosurgery showed improvements in tremor from blinded, videotaped ratings at 1 year. Radiosurgical thalamotomy has not been embraced because intraoperative validation is not possible, the effects are delayed, and there are theoretical concerns about radiation side effects, secondary neoplasia, and a less-sharp dose gradient. Our study has several limitations. First, the procedures were all performed unilaterally.
Although unilateral focused ultrasound thalamotomy improved total tremor scores by 47% in the study cohort, there was no reduction of ipsilateral tremor and only minimal improvement in axial tremors of the head, neck, and voice. Second, some patients may be reluctant or unwilling to undergo MRI studies or it may be unsafe for them to do so. Third, lesioning procedures require a balance between the size of the lesion and the risk of adverse effects, since larger lesions are expected to have more enduring efficacy but a greater incidence of side effects. Finally, transcranial delivery of focused ultrasound was difficult to achieve in five of the study patients, probably because of the frequency and other properties of the acoustic wave, as well as individual cranial characteristics.
Additional research is needed to address this issue. In conclusion, our study showed that MRI-guided focused ultrasound thalamotomy reduced hand tremor and improved the quality of life in patients with essential tremor.
Side effects included sensory and gait disturbances. The benefits and risks of focused ultrasound thalamotomy performed in a carefully controlled clinical trial may differ from the benefits and risks with routine practice in diverse clinical settings. Supported by InSightec, the Focused Ultrasound Foundation, and the Binational Industrial Research and Development (BIRD) Foundation. Provided by the authors are available with the full text of this article at NEJM.org.
Elias reports receiving grant support from InSightec and the Focused Ultrasound Foundation; Dr. Lipsman, receiving fees from the Focused Ultrasound Foundation for serving on a steering committee; Dr. Ghanouni and Dr. Butts Pauly, receiving grant support from InSightec and GE Healthcare; Dr. Hynynen, receiving royalties from a patent related to ultrasound therapy (US6770031 B2); Dr. Lozano, receiving grant support from InSightec; Dr.
Gwinn, receiving teaching fees from NeuroPace and Boston Scientific; Dr. Cosgrove, receiving consulting fees from InSightec; and Dr.
Taira, receiving lecture fees from Daiichi-Sankyo, Eisai, GlaxoSmithKline, Otsuka, Pfizer, Hisamitsu, Dainippon-Sumitomo, Takeda, and Kyowa-Hakko, and grant support from St. Jude Medical. No other potential conflict of interest relevant to this article was reported. We thank Rodger Elble, M.D., Ludy Shih, M.D., Peter Lewitt, M.D., Raj Pahwa, M.D., Dan Tarsy, M.D., and Theresa Zesiewicz, M.D., of the Tremor Research Group for performing videotape tremor ratings, and Vera Hashem, Ph.D., for coordinating the core laboratory. References • 1 Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor.
Mov Disord 2010;25:534-541 • 2 Ho AL, Erickson-Direnzo E, Pendharkar AV, Sung CK, Halpern CH. Deep brain stimulation for vocal tremor: a comprehensive, multidisciplinary methodology.
Neurosurg Focus 2015;38:E6-E6 • 3 Elias WJ, Shah BB. JAMA 2014;311:948-954 • 4 Lorenz D, Schwieger D, Moises H, Deuschl G. Quality of life and personality in essential tremor patients. Mov Disord 20-1118 • 5 Louis ED, Machado DG. Tremor-related quality of life: a comparison of essential tremor vs.
Parkinson’s disease patients. Parkinsonism Relat Disord 2015;21:729-735 • 6 Schneier FR, Barnes LF, Albert SM, Louis ED. Characteristics of social phobia among persons with essential tremor. J Clin Psychiatry 2001;62:367-372 • 7 Deuschl G, Bain P, Brin M; Ad Hoc Scientific Committee.. Consensus statement of the Movement Disorder Society on Tremor.
Mov Disord 1998;13:Suppl 3:2-23 • 8 Deuschl G, Raethjen J, Hellriegel H, Elble R. Treatment of patients with essential tremor.
Lancet Neurol 2011;10:148-161 • 9 Findley LJ, Calzetti S. Double-blind controlled study of primidone in essential tremor: preliminary results. Br Med J (Clin Res Ed) 1982;285:608-608 • 10 Winkler GF, Young RR. Efficacy of chronic propranolol therapy in action tremors of the familial, senile or essential varieties. N Engl J Med 1974;290:984-988 • 11 Zesiewicz TA, Elble R, Louis ED, et al. Practice parameter: therapies for essential tremor: report of the Quality Standards Subcommittee of the American Academy of Neurology.
Neurology 20-2020 • 12 Benabid AL, Pollak P, Seigneuret E, Hoffmann D, Gay E, Perret J. Chronic VIM thalamic stimulation in Parkinson’s disease, essential tremor and extra-pyramidal dyskinesias. Acta Neurochir Suppl (Wien) 1993;58:39-44 • 13.
「認知帕金森」('Parkinson's awareness')活動的標誌,標誌中央為一朵紅色鬱金香。 帕金森氏症帶來龐大的社會成本,實際金額因方法問題及國別差異而難以計算 。英國每年花費於帕金森氏症的金額估計在4.49億至33億之間;美國則約為230億美元,平均每名病患每年花費約1萬美元 ,其中最大一部份用於 ( 英語 : )和護理之家,其次則為藥物花費 。2006年中國上海的統計指出該地每名帕金森氏症患者每年平均花費7679人民幣,這個數目是當地平均所得的一半,其中最大的開銷來自藥物花費 。除了直接成本外,帕金森氏症也帶來鉅額的間接成本,例如患者的生產力下降,並連帶加重照顧者的勞力與經濟負擔,且影響到雙方的生活品質 。 倡議 [ ] 為提升公眾對於該疾病的重視,歐洲帕金森氏症協會將詹姆士帕金森的生日(4月11日)訂為每年的世界帕金森日(World Parkinson's Day) 。2005年,國際組織將紅色鬱金香選為本疾病的象徵,原因是一位荷蘭的園藝家威爾德(J.W.S. Van der Wereld)將其於1981年培育出來的命名為「詹姆士帕金森鬱金香」 。 ( 英語 : )自1982年起每年提供1.8億美元,贊助帕金森氏症相關的照護、研究及服務 。 ( 英語 : )由威廉布拉克(William Black)在1957年所創辦,自成立以來已捐助了1.15億美元於相關研究、5000萬美元於教育及推廣計畫 。其他還包含1961年成立的 ( 英語 : ) ,以及1992年成立的 ( 英語 : ) 。 著名病例 [ ].